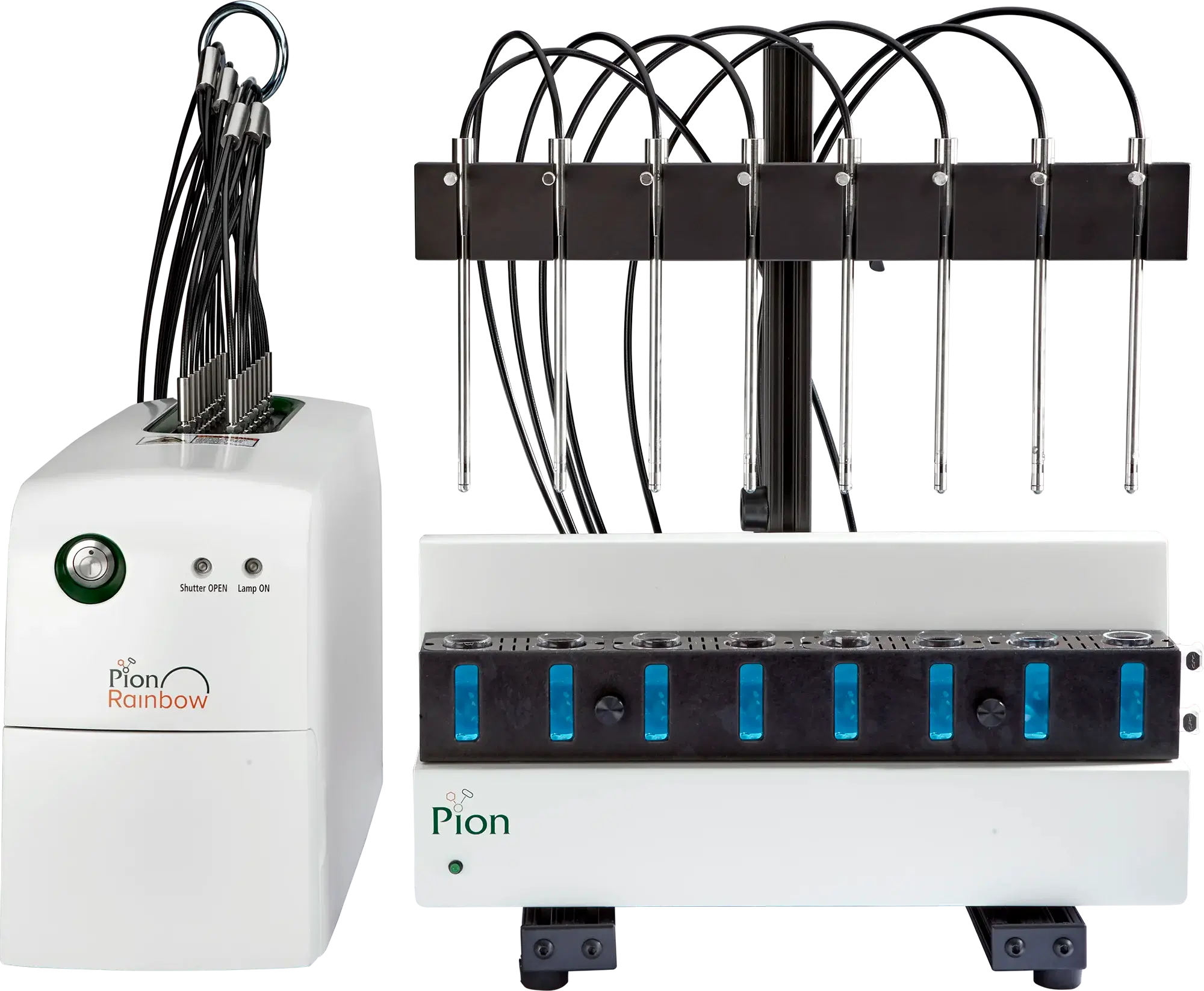
MicroFLUX
In-vitro assessment of dissolution-absorption processes in a low-volume environment for early-stage ranking of in-vivo outcomes.






In-vitro assessment of dissolution-absorption processes in a low-volume environment for early-stage ranking of in-vivo outcomes
Low Volume Dissolution-Absorption testing
Utilizing the Pion Rainbow for in-situ real-time measurement
Simultaneous assessment of drug substance
Combine Dissolution and Absorption measurements into a single assay
In-vitro assessment of dissolution-absorption processes in a low-volume environment for early-stage ranking of in-vivo outcomes
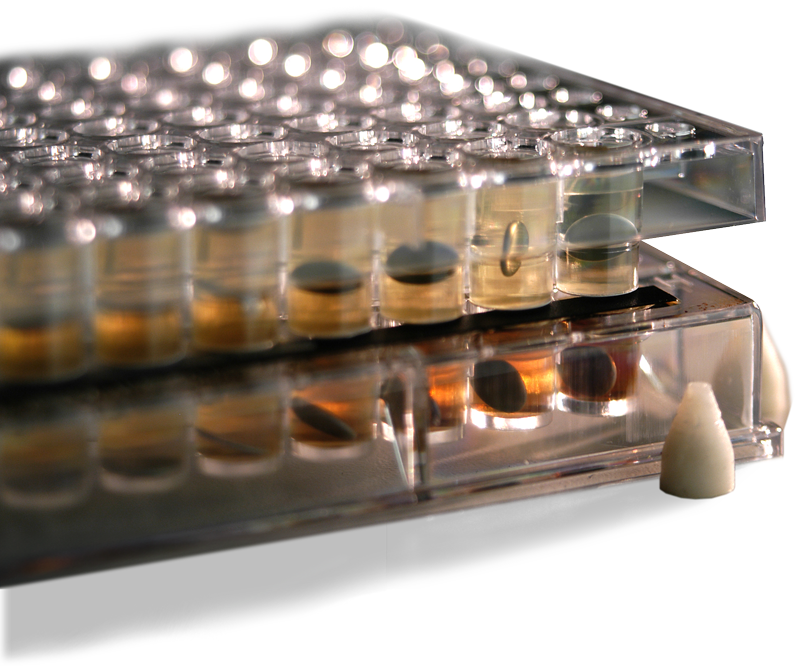
Low-volume absorption chamber
The MicroFLUX™ device consists of a low-volume absorption chamber separated from a low-volume donor chamber with a biomimetic gastrointestinal tract permeation membrane. Compatible with a wide range of aqueous and biorelevant medias, the MicroFLUX can be used for rapid evaluation of sample performance under a variety of conditions with a low media and sample burden.
The impact of excipients on drug behavior when conducting these simultaneous dissolution-absorption studies provides an understanding of the complex interplay between solubility, permeability, and dissolution rate in-vitro and establishes the MicroFLUX as a valuable tool for ranking in vivo outcomes.












