In vitro testing of Subcutaneous (SC) monoclonal antibodies (mAbs)
Ensuring absorption and bioavailability of your subcutaneous (SC) mAb therapy is critical for drug effectiveness. For a long time, scientists’ primary method for testing formulations was using animals

In 2025, the FDA has released new guidance on testing subcutaneous (SC) monoclonal antibodies (mAbs) and lays out support for in vitro New Approach Methodologies (NAMs) to reduce animal testing for SC monoclonal antibody (mAb) drugs.
This shift is historic, cutting down on ethically challenging, time-consuming, and costly animal studies, while accelerating access to life-saving medicines. At Pion, we’ve been pushing this approach since our inception and we’re thrilled the regulatory community will be joining us on this journey.
We ARE the SubCutaneous Injection Site SimulatOR – SCISSOR N3™!
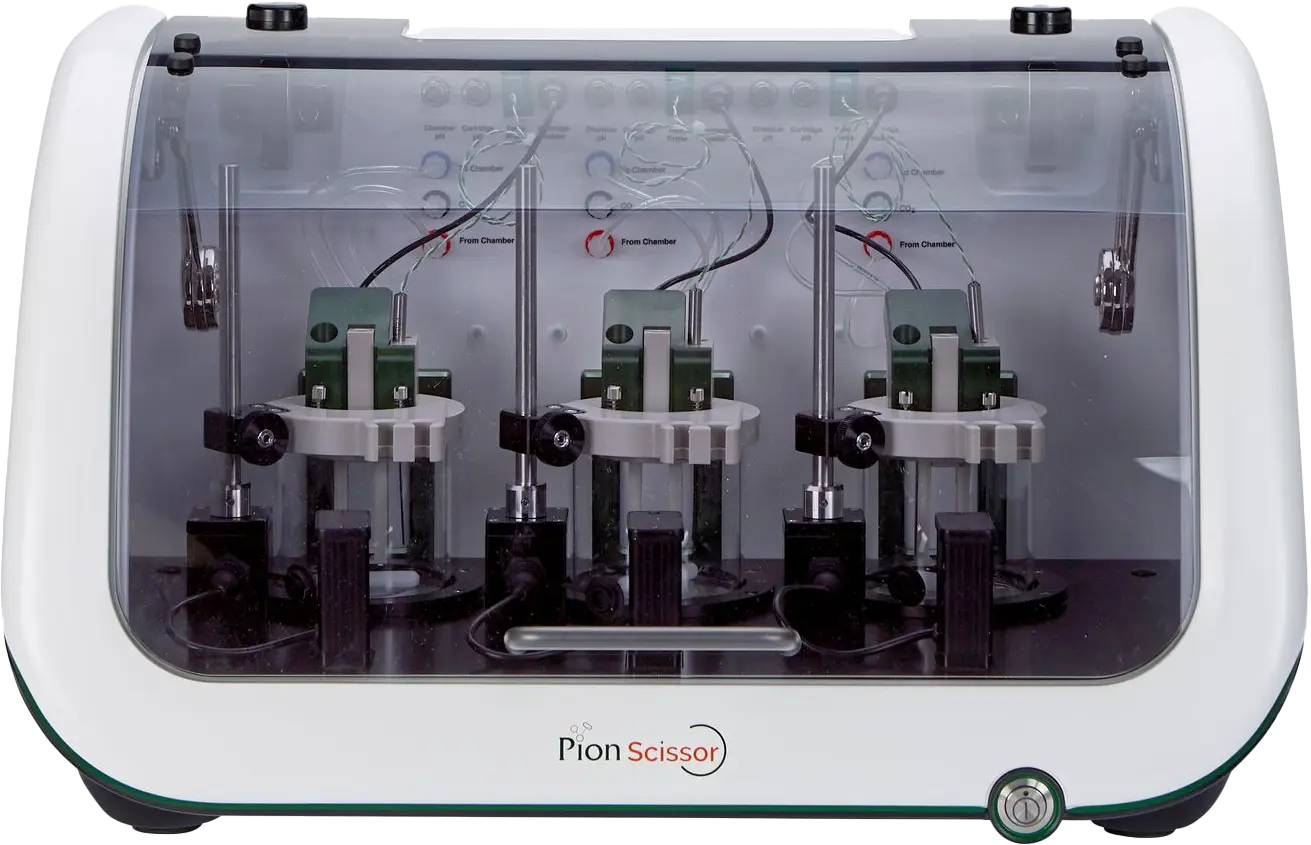
Pion developed and validated SCISSOR N3 using mAbs 10 years ago, specifically to simulate SC drug behavior in vitro, enabling informed prediction and comparison of drug performance in vitro, helping reduce animal testing today, not years from now.
The FDA’s shift is a leap forward—and we’re already there.
See SCISSOR N3 in action!
Listen to Pion Scientist Conor Gomes summarize a recent publication by AstraZeneca in the Journal of Controlled Release where they used SCISSOR to investigate in vitro – in vivo correlations.